|
home |
index |
units |
counting |
geometry |
algebra |
trigonometry |
calculus |
functions
analysis | sets & logic | number theory | recreational | misc | nomenclature & history | physics |
NOTICE:
The text quoted below was transcribed by Dr. Gerard P. Michon from a copyrighted
For those who want some proof that physicists are human, the proof is in
the idiocy of all the different units which they use for measuring energy. The Character of Physical Law (1967) R.P. Feynman [ 1964-11-12 video ] Before I begin the lecture [on spacetime], I wish to apologize for something that is not my responsibility: Physicists and scientists all over the world have been measuring things in different units, and causing an enormous amount of complexity. As a matter of fact, nearly a third of what you have to learn 1 consists of different ways of measuring the same thing, and I apologize for it. It's like having money in francs, and pounds, and dollars... with the advantage over money that the ratios don't change, as time goes on. For example, in the measurement of energy, the unit we use here is the joule (J), and a watt (W) is a joule per second. But there are a lot of other systems to measure energy. There are at least three different ones for engineers, which I have listed here.2
The physicists do something else when they want to talk about the energy
of a single atom, instead of the energy of a gross amount of material.
The reason is, of course, that a single atom is such a small thing that to talk
about its energy in joules would be inconvenient.
But instead of taking a definite unit in the same system
(like
Now, there is an additional unit that the physical chemists use, the kilocalorie per mole (kcal/mol), and 23 of those is an electronvolt per atom. [23 kcal = 96500 J] Finally, unfortunately, you have another system for measuring masses. The mass of an atom, from a chemist's point of view, is given by the mass of a mole of these atoms. For example, the mass of carbon-12 is called 12 "atomic mass units" (u), because a mole of carbon-12 "weighs" 12 grams (or rather "has 12 grams of mass"). One atomic mass unit represents one gram for every mole of objects, one gram per mole. We can measure that in electronvolts also. "You can't measure mass in electron volts!" 5 Sure you can, because of the relation E = mc2 ... It is useful to know how much energy corresponds to the consumption of one atomic mass unit of material: That turns out to be about 931 million electronvolts (MeV). Incidentally, the rest mass of a proton is 938 MeV, while the rest mass of an electron corresponds to 0.511 MeV. The number 938 differs from 931, because a proton has a mass of about 1.008 amu. I am sorry about the confusion produced by all these systems of units. I left out, obviously, a large number of different things. For example, when measuring luminous 6 energy, the lumen (lm) is used, which corresponds to about 1.5 mW of power in the "most visible" light, around 5500 Å (ångströms). It's all very annoying, but don't worry about it now. When you need to measure light, just look up in a book what a lumen is. That's an unfortunate fact that we measure things in a whole series of different kinds of units. This causes a lot of confusion. It's too bad, but I have already apologized, and there is nothing else I can do... Richard P. Feynman (1961)
Notes : 1 Feynman may well be overstating the case, but the opposing understatement is so widespread that we decided to feature this statement as a subtitle for this web page... 2 Consistent "engineering" systems of mechanical units are listed below. The units of energy and power advocated by Feynman for use at CalTech, the joule (J) and the watt (W), are metric (SI) units. The other systems are now obsolete, or are being deprecated: 3 The CGPM finally adopted this definition of the mole in 1971. It is based on the "unified" definition of the "atomic mass unit" as 1/12 the mass of a Carbon-12 atom, which was adopted at the 10th General Assembly of the International Union of Pure and Applied Physics (Ottawa, 1960) and subsequently by the International Union of Pure and Applied Chemistry in 1961. The unified unit (symbol "u") replaced two earlier competing definitions, both using the symbol "amu", which were based on different aspects of oxygen: The physical amu was defined to be 1/16 of the mass of the Oxygen-16 atom, while the larger chemical amu was 1/16 of the average mass of an atom of natural oxygen, with the isotopic composition found on Earth (99.759% O-16, 0.037% O-17, 0.204% O-18). The unified amu (u) is only 0.004% more than this chemical amu. Thus, the chemists got to keep the value of their amu, while the physicists got their wish in having the atomic mass unit defined in terms of a given nucleus, not an isotopic mixture. The symbol for the unified amu is "u". A chemical amu was 0.999958 u, a physical amu was 0.999687 u. [See sizes.com]. The unified atomic mass unit (u) is increasingly referred to as a "dalton" (symbol Da) in honor of John Dalton (1766-1844). That name was adopted by the IUPAC in 1993, by the CIPM in 2003, by the IUPAP in 2005 and by ISO in 2009. 4 Moving a mole of electrons through a potential of one volt involves an energy equal to a mole of electronvolts. Expressed in joules, this equals one volt multiplied by the charge of a mole of electrons, expressed in coulombs (Faraday's constant). 5 To remove this objection, the "Mev of mass" is now best abbreviated Mev/c2. Similar notations are used for other mass units obtained from various energy units. 6 In the spoken lecture, Feynman wrongly used the qualifier "radiant", instead of the term "luminous" which properly qualifies electromagnetic radiation measured with the spectral sensitivity of the human eye under normal "photopic" conditions (when the eye adapts to darkness, it has a different spectral response, which is called "scotopic"). Luminous power --often called "luminous flux"-- is expressed in lumens, whereas the corresponding radiant power would simply be measured in watts. The qualifier "bolometric" may be used for radiant power, to insist that radiant measurements are meant to include all energies at all frequencies with the same weight, which is not the case for luminous measurements.
We are proud to have this page belong to the Feynman Webring, which connects a number of fine pages which perpetuate the legacy of Richard Feynman in various ways. Some are more controversial than others:
At this writing, the next site
in this ring happens to feature an essay where
James G. Gilson
presents his own formula (involving two integer parameters)
for the value of the so-called
Fine-Structure Constant
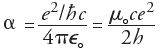 Sommerfeld's Fine-Structure Constant may be viewed as the only free parameter in QED, the relativistic quantum theory of the interactions between electrons and photons (for which Feynman, Schwinger and Tomonaga shared the 1965 Nobel Prize). In QED, the coupling constant's effective limit is simply the square root of a, and Feynman was understandably annoyed that QED was thus based on an unexplained numerical value. He expressed the wish that a deeper understanding of Nature would eventually explain that value and/or allow it to be expressed in terms of known constants, like p or e. Before and after Feynman, others have tried to guess such a relation, possibly hoping that it could be a clue to whatever more general theory may underly our current understanding of the laws of Nature. Around 1923, Sir Arthur Eddington (1882-1944) "proved" a to be 1/136, in agreement with early rough estimates. When subsequently faced with incompatible experimental data, he amended the "proof" to show that the value had to be 1/137, so that Punch magazine dubbed him Sir Arthur Adding-One. See 137 by Charles C. Mann, or look up the description by Robert Munafo of the so-called Eddington number [the outrageously asserted total number of electrons in the Universe, as the inverse of the fine structure constant multiplied by a power of two]. The two integers in Dr. Gilson's dubious formula may be tuned to accurately reflect modern experimental data only up to a point: The pair (137,25) was the best match for the midrange of the previous (1986) CODATA value of a [namely Gilson's formula and its justifications are pseudoscientific. Gilson is [at best] guilty of wishful thinking when he presents his formula as a generally accepted fait accompli. This is simply not so. You've been warned; you may proceed (http://www.fine-structure-constant.org/page5A.html).
Photos from ESVA (AIP) | UMD photos | Dissertation | Wikipedia Videos :Clips | BBC Interviews: Uncertainty | Flower | Honors | Inertia | ChessBBC Horizon Interviews: 1 | 2 | 3 | 4 | 5 "Atom" (BBC): 1 | 2 | 3 Messenger Lectures (Cornell University, November 1964) "The Character of Physical Law" : 1-Gravitation | 2-Mathematics | 3-Conservation | 4-Symmetry | 5-Past & Future | 6-Quantum Strangeness Minus Three (BBC Horizon, 1964) 1 | 2 | 3 Take the world from another point of view (1972) 1 | 2 | 3 | 4 QED made simple (1979 Conference) | Horizon - "The pleasure of finding things out" (1983) "Fun to Imagine" (1983) Jiggling | Fire | Rubber | Why? Electricity | Mirror | Train | Seeing | Stuff | Stars | Think | Imagine Connection between Spin and Statistics (1986 Dirac Memorial Lecture, about the spin-statistics theorem) The Reason for Antiparticles, Part II, Part III, Part IV, Part V Remembering Richard Feynman (Caltech, 2015-05-14). Growing Up Feynman (11:47) by Michelle Feynman (Caltech, 2018-05-11). John Wheeler | Hans Bethe | Freeman Dyson + road trip | Murray Gell-Mann | Leonard Susskind Feynman's lost lecture (21:43) by Grant Sanderson (2018-07-20). |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||


